-
Needle-free Vaxxas HD-MAP patch offers potential platform to dramatically improve vaccination in pandemic response by producing more patient-doses from limited quantities of vaccine, enabling rapid vaccine distribution without refrigeration, and creating a novel option for self-administration.
-
Initial BARDA funding focuses on clinical demonstration using pandemic influenza vaccine; Vaxxas investigating opportunities to improve performance of other pandemic vaccines including against COVID-19, as well as a broad range of non-pandemic infectious disease vaccines.
CAMBRIDGE, Mass. & BRISBANE, Australia --- Vaxxas Inc. a clinical-stage biotechnology company commercializing a novel vaccination platform, today announced a $22 million United States government award to support the deployment of Vaxxas’ proprietary HD-MAP technology platform in response to pandemic threats to public health. The award is funded through the Biomedical Advanced Research and Development Authority (BARDA), part of the Office of the Assistant Secretary for Preparedness and Response (ASPR) at the U.S. Department of Health and Human Services, and is aimed at advancing clinical demonstration of Vaxxas’ proprietary HD-MAP patch for pandemic influenza. Aligned with this approach, Vaxxas is actively investigating opportunities to improve performance of other pandemic vaccines including against COVID-19, as well as a broad range of non-pandemic infectious disease vaccines.
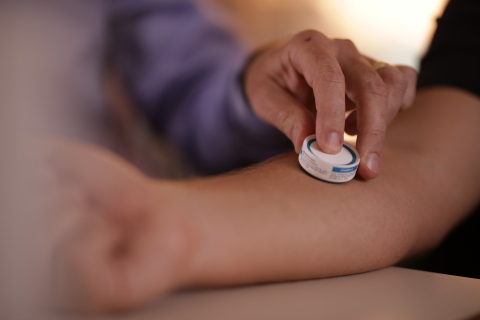
Vaxxas HD-MAP Application (Photo: Business Wire)
Under the terms of the $22 million award from BARDA, contract number 75A50120C00180, Vaxxas will perform a phase 1 clinical study using Vaxxas’ high density micro-array patch (HD-MAP) delivering pandemic influenza vaccine to more than 400 people using both unadjuvanted and adjuvanted vaccine formulations. The total cost of this project is estimated to be $24.1 million of which 8.5 percent or $2.1 million will be contributed by Vaxxas. Pandemic influenza vaccine was selected for this clinical validation study to comprehensively baseline the immune responses and safety of the novel HD-MAP vaccination platform when used for pandemic preparedness and response. Vaxxas’ HD-MAP platform is attractive for pandemic response as it has clinically shown the potential to transform vaccine delivery with lower dose requirements, enabling more patient-doses to be manufactured more quickly from limited vaccine supply. In addition, the Vaxxas HD-MAP patch enables faster immune onset kinetics and higher antibody responses, potentially resulting in more rapid and durable disease protection; and vaccines that do not need refrigeration, enabling rapid distribution using population-scale logistics such as USPS, UPS, FedEx and more. Unlike needle/syringe, the HD-MAP patch is easy to apply, with studies currently underway to demonstrate self-administration.
In response to demands from collaborators such as Merck, The World Health Organization and the Bill and Melinda Gates Foundation, Vaxxas has prototyped a compact manufacturing system designed to be capable of delivering more than 250 million vaccine doses per year.
“We are excited to partner with BARDA to rapidly deploy Vaxxas’ HD-MAP technology, which holds the promise of significantly improving pandemic response with needle-free vaccine delivery that is more effective and readily accepted,” Vaxxas Chief Executive Officer David L. Hoey stated. “Having validated our HD-MAP technology in clinical studies at commercial scale, we stand ready to be among the leading innovators who can solve critical challenges to global pandemic health crises, going beyond and enhancing the capabilities enabled by promising vaccines.”
Vaxxas has published comprehensive data from its clinical programs, including the largest microarray patch clinical vaccination study ever performed which was published in PLoS. In this first clinical microarray patch study to show dose sparing against standard intramuscular injection with comparable immune responses at a 1/6 dose, Vaxxas’ HD-MAP immune response was shown to be significantly higher and have faster onset than by intramuscular injection at comparable doses. Vaxxas HD-MAP is a 9x9mm array of thousands of very short (~250µm) projections, invisible to the naked eye, coated with vaccine. Application of the HD-MAP to the skin delivers vaccine to abundant populations of immune cells. Vaccine on HD-MAP has been shown to be stable for 12 months at temperatures as high as 40oC.
More than 750 million vaccinations are given routinely worldwide each year. In the case of pandemic response, billions of vaccinations may need to occur in a variety of settings. Vaxxas’ HD-MAP has the potential to create new needle-free vaccine products with enhanced immune response that would be designed for improved safety, storage, and distribution logistics to extend the reach of vaccines and improve vaccination efficiency and effectiveness.


