Hunt Valley, MD -- Pharmaceutics International, Inc. (Pii), a contract development and manufacturing organization (CDMO), will include Daikyo Crystal Zenith® (CZ) container-closure components, offered by West Pharmaceutical Services, Inc. (West), as part of its aseptic fill-finish capabilities across vials, syringes and cartridges.
Recognizing the growing demand for high-quality and advanced containment systems, as well as small and medium-sized batches, Pii has enabled its AST GeniSYS® R sterile fill-finish system to handle West’s CZ platform to provide our customers with more options, greater flexibility, and strategies to de-risk their product development. Pii’s expertise and experience in developing and manufacturing high-value, complex molecules will be greatly improved with the addition of CZ polymer containers that offer high-quality standards, design customizations, and flexibility, along with physical and chemical benefits.

Pii’s initial implementation will be filling ready-to-use (RU) syringes and cartridges in its aseptic fill-finish processes. According to Dr. Kurt Nielsen, President and CEO of Pii, “This new capability supports our strategic objectives of providing flexible solutions for our clients as we help them overcome the most significant development and production challenges in advancing complex parenteral formulations.”
“Integrating CZ polymer containers and delivery devices with the agile capabilities of the AST GeniSYS R offers a vertically integrated set of solutions that are key to Pii’s ability to streamline our pharmaceutical development services and deliver quality results faster,” says Devan Patel, Senior Director, Project Management at Pii.
For a typical project, pre-sterilized delivery devices will arrive at our facilities in a syringe nesting system using bulk nested trays. This minimizes the risk of contamination and shortens project lead time.
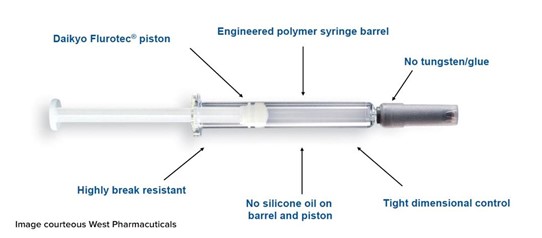
Why Choose Cyclic Olefin Polymer Over Glass?
West Pharmaceutical's Daikyo Crystal Zenith® container-closure components are made of a cyclic olefin polymer. Daikyo Crystal Zenith® containers come pre-sterilized using bulk nested trays as a syringe nesting system. This minimizes the risk of contamination and shortens project lead time.
Additional Benefits to Cyclic Olefin Polymer
- Outstanding barrier properties outperforming many plastics
- Better oxygen permeability properties than many of its peer polymers such as polypropylene, polystyrene, and polycarbonate
- Protects the next most deleterious material to many drug products: water vapor, a plus for any non-aqueous and dry drug products
- Good candidate for moisture-sensitive products. This property is inherent to Crystal Zenith, thanks to its nonpolar material properties.
- Unlike glass, Crystal Zenith is free of inorganic extractable (Data indicates CZ has low organic extractables, especially compared to other plastic packaging materials.)
- Crystal Zenith is equivalent to glass and superior to polypropylene regarding sorption of these model preservatives, keeping the excipient material of the drug product
West Pharmaceutical Services, Inc. is a leading global manufacturer in the design and production of technologically advanced, high-quality, integrated containment and delivery systems for injectable medicines. We are a trusted partner to the world’s top pharmaceutical and biotechnology companies— working by their side to improve patient health. www.westpharma.com Crystal Zenith® is a registered trademark of Daikyo Seiko, Ltd. Crystal Zenith® technology is licensed to West Pharmaceutical Services, Inc. from Daikyo Seiko, Ltd