Ease of molecular synthesis and portfolio diversity enables small molecules to be consistently victorious compared to other drug modalities. A staunch focus on creativity in chemical space and enriched understanding of drug parameters are surefire ways to spur innovation and a concomitant cure.
Small Molecules Rule The Therapeutic Landscape
In the realm of chemical modalities, small molecules have consistently been a lucky charm. Over 90% of FDA-approved drugs are small molecules. A record number of 45 new molecular entities (NMEs) approved by FDA in 2015 – and about 16 NMEs until July 2016 – accentuate how the properties of a small molecule can be tailored to fit patient needs. In this article, I highlight two promising small molecules (see Figure 1 for structures) currently in clinical trials. The first part of the article investigates the role of CPP-115 (completed phase I) in epilepsy, whereas the second part focuses on the treatment of acute myeloid leukemia (AML) using quizartinib (in phase III).
Cpp-115: An Investigational Drug For Epilepsy
The fact that 1 in 12 people will have a seizure in their lifetime raises alarming signals to mitigate, prevent and cure epilepsy. The etiology is still unclear, but one of the pharmaceutical strategies to treat seizures is to replenish the local concentrations of GABA (gamma-aminobutyric acid, an inhibitory neurotransmitter in the human brain) that is degraded by an enzyme called GABA aminotransferase (GABA-AT). Mere consumption of GABA capsules is not effective, due to its inability to cross the blood-brain barrier (BBB). Therefore, an alternative strategy that involved stopping the function of GABA-AT was envisioned. Sabril is a first-in-class, FDA-approved antiepileptic drug; however, its daily dosage limit (1g – 3g) and adverse side effects, which include vision defects, call for further innovation.
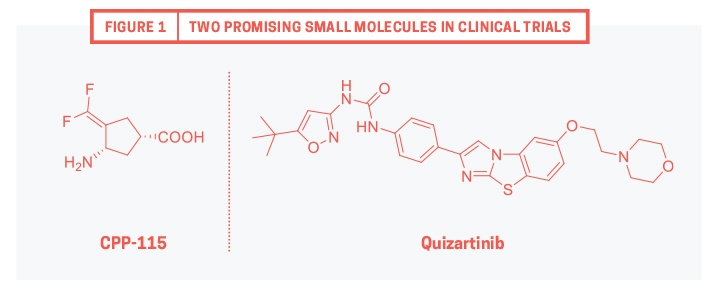
Prof. Richard Silverman and his lab members at Northwestern University embarked on a scientific journey to identify BBB-penetrating antiepileptic compounds that would not cause visual defects. Through computational modeling and several cycles of optimization they discovered CPP-115 (chemical name: (1S,3S)-3-amino-4-difluoromethylene-1-cyclopentanoic acid; kinact/KI = 52 mM.min-1.)1 Mechanistically, CPP-115 binds to GABA-AT, undergoing product transformation that kills GABA-AT’s function. In rat studies, CPP-115 suppressed spasms at a much lower dose (0.1 mg/kg) than Sabril (>200 mg/kg) and exhibited better tolerance without visual defects.
CPP-115 (licensed to Catalyst Pharmaceuticals) elicited no cross-inhibition. It is metabolically more stable, with favorable PK characteristics (including rapid absorption and clearance). In a randomized, double-blind, single ascending dose phase I(a) study, CPP-115 was very well tolerated in all six doses (n=55 patients; maximum dose 500 mg, therapeutic dose 80 mg/day).2 Phase I(b) studies conducted in double-blind, placebo-controlled conditions demonstrated the safety and tolerability of CPP-115 in healthy volunteers. Intriguingly, an increase in brain GABA levels (150% to over 200%) was detected, accentuating CPP-115’s antiepileptic potential.2 Further clinical trials are currently in progress. CPP-115, with 12 years of unexpired patent life, has been granted orphan-drug designation in both the U.S. and EU for treating infantile spasms.
Nature has a penchant for small molecules and so do pharmaceutical companies!
Quizartinib — An Investigational Drug For Acute Myeloid Lymphoma
AML is a type of cancer characterized by uncontrolled production of myeloblasts (a type of white blood cell), red blood cells and platelets. This hematologic malignancy starts in the bone marrow, but quickly invades the blood and other parts of the body, including the liver, spleen and brain. A quarter of AML patients harbor FLT3-ITD mutation, an even more aggressive form of the AML. Current treatment options, including induction chemotherapy and transplantation, have met with mixed results because most patients display a high propensity for relapse after remission.
Bhagwat and his team at Ambit Biosciences (acquired by Daiichi Sankyo) attempted to stop this “backward sledding.” The first-generation FLT3 inhibitors identified by them were potent; however, problems associated with solubility and PK precluded these inhibitors from further advancement. Bhagwat’s team quickly transformed them into their flagship compound by appending water-soluble groups and clipping off an undesired carboxamide. Quizartinib (chemical name: 1-(5-(tert-Butyl)isoxazol-3-yl)-3-(4-(7-(2-morpholinoethoxy)benzo[d]imidazo[2,1-b]thiazol-2-yl)phenyl)urea; IC50 = 1 nM), a highly potent and selective FLT3 inhibitor, displayed excellent PK profile in rat and mice models, and killed tumors implanted on mice.3
In a dose escalation phase I study (12 to 450 mg/day) involving 76 patients, quizartinib exhibited safety, acceptable toxicity and clinical activity against both relapsed and refractory AML conditions.4 In phase II (two cohorts), quizartinib sustained a high degree of activity in over 50% of FLT3-ITD(+) patients who showed composite complete response (CRc). About 50% of patients who were refractory (i.e., have not responded yet to prior AML therapy) achieved CRc as well.5 Phase III results to assess the efficacy of quizartinib as a solo therapy, as well as in combination with other chemotherapeutic drugs, are in progress.
Epilogue
Out of 1060 theoretically possible small molecules, a majority are still unexplored. The small molecules presented in this article are highly innovative and are ambitiously expected to become drugs. Nature has a penchant for small molecules and so do pharmaceutical companies!
References
- Silverman, Richard B. “The 2011 E. B. Hershberg Award for Important Discoveries in Medicinally Active Substances: (1S, 3S)-3-amino-4-difluoromethylenyl-1-cyclopentanoic acid (CPP-115), a GABA Aminotransferase Inactivator and New Treatment for Drug Addiction and Infantile Spasms.” Journal of Medicinal Chemistry 55.2 (2012): 567-575. Web.
- Catalyst Pharmaceuticals Announces Top-Line Results in Phase 1(b) Trial of CPP-115. Globe Newswire. 16 Dec. 2015. Web.
- Chao, Qi, Kelly G. Sprankle, Robert M. Grotzfeld, Andiliy G. Lai, Todd A. Carter, et al. “Identification of N-(5-tert-Butyl-isoxazol-3-yl)-N’-{4-[7-(2-morpholin-4-yl-ethoxy) imidazo[2,1-b][1,3]benzothiazol-2-yl]phenyl}urea dihydrochloride (AC220), a Uniquely Potent, Selective, and Efficacious FMS-Like Tyrosine Kinase-3 (FLT3) Inhibitor.” Journal of Medicinal Chemistry 52.23 (2009): 7808-16. Web.
- Cortes, Jorge E., Hagop Kantarjian, James M. Foran, Darejan Ghirdaladze, Mamia Zodelava, et al. “Phase I Study of Quizartinib Administered Daily to Patients with Relapsed or Refractory Acute Myeloid Leukemia Irrespective of FMS-Like Tyrosine Kinase 3 - Internal Tandem Duplication Status.“ Journal of Clinical Oncology 31.29 (2013): 3681-7. Web.
- Astellas and Ambit Announce Data Presentations Highlighting Quizartinib at the American Society of Hematology 54th Annual Meeting. PRNewswire. 6 Nov. 2012. Web.

