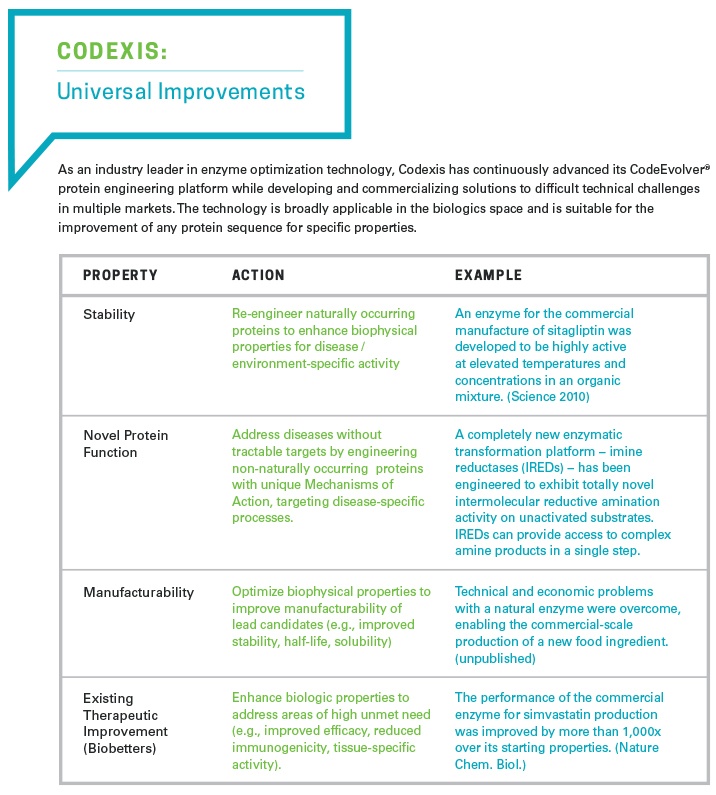
By applying its CodeEvolver® directed evolution technology, Codexis has been improving enzymes for specific functions in challenging environments for well over a decade, using high-throughput gene synthesis, biochemical screening and advanced data analytics. Recently we have demonstrated that this technology can impact biologic drug candidate discovery and optimization. By applying the same core technology and optimization philosophies successfully demonstrated in engineering biocatalysts for the synthesis of small-molecule active pharmaceutical ingredients (APIs), we are now fundamentally changing the properties of biologics to generate novel and potentially more effective drug candidates.
The first step in CodeEvolver®-driven enzyme engineering involves defining the desired enzyme performance characteristics that are projected to address the specific unmet need. Then, application of the CodeEvolver® technology over iterative rounds of laboratory evolution leads to an engineered, novel, high-performance enzyme, which meets the original design criteria. Codexis then demonstrates the manufacturability and effectiveness of the enzyme in the end-use application by producing the enzyme in its proprietary expression platforms and performing relevant efficacy studies.
This approach is highly attractive for addressing the shortcomings of existing enzyme replacement therapies. Historically, native human enzymes (mostly produced as recombinant enzyme) formed the basis for ERTs, sometimes with some minor tweaks through cell line engineering, chemical modification, and genetic engineering. Genetic engineering is generally regarded as highly challenging, as a result of the near-infinite number of possible changes that can be made in a protein, with related concerns around immunogenicity. The CodeEvolver® technology platform renders these challenges addressable and, most important, solvable.
The CodeEvolver® technology platform is broadly applicable in the biologics space and can be used to improve any protein sequence for specifically defined properties. Enzymes are a subset of the protein world and thus the initial focus at Codexis has been on enzyme replacement therapies, given the natural fit with the company’s biocatalyst experience and expertise.
In addition, while nucleic acid-based treatments — gene and mRNA therapies — are competitive technologies opposite pure enzyme replacement therapies, the improved protein sequence developed using CodeEvolver® technology can be administered as a polypeptide, DNA or mRNA. Consequently, protein engineering has the potential to play a significant role in the advancement of this segment of the orphan drug market.
The overall focus for Codexis is on the development of biotherapeutics that address unmet medical need and require our capabilities for improving protein properties, providing novel functionality, and potentially reducing immunogenicity. By doing so, we expect to be able to showcase the effectiveness of the CodeEvolver®-directed evolution technology platform and demonstrate that this technology is broadly applicable to the development of novel, effective biologic drugs.
Proven Technology Ready To Penetrate An Increasing Number Of Applications
Codexis’ core business of developing enzymes for small-molecule manufacturing has been proven at scale many times. Our experience in improving the activity, manufacturability and stability of enzymes aligns perfectly with the need to address the suboptimal efficacy and stability of current enzyme replacement therapies, resulting in a direct synergy between our experience and competence and the unmet needs in the therapeutic enzyme market. Further expansion of the application of our core expertise from enzymes towards all polypeptides is an obvious next step. We are developing an internal pipeline of candidates around high-priority targets and five enzymes are progressing through this program. Ultimately, the intention is to out-license these assets at appropriate stages in clinical development.
Concomitant with our efforts aimed at developing our own pipeline of biotherapeutic candidates is the creation of novel proteins for other targets requested by biopharmaceutical companies. In this case, we are seeking experienced partners with significant challenges in their protein development programs and who are interested in collaboratively exploring the application of our CodeEvolver®-directed evolution platform technology to seek a solution. For collaborative projects, Codexis expects to extend beyond enzymes to, for example: Fc-fusion proteins; antibody-drug conjugates (ADCs); monoclonal antibodies (mAbs); etc. These partnerships will involve flexible technical and program management approaches designed to suit the specific market and application needs of each project.
Lead Program: An Oral Enzyme Therapy For Treatment Of PKU
Our lead asset is an enzyme for potential treatment of phenylketonuria (PKU), a prevalent orphan disease that results from the absence of the phenylalanine hydroxylase enzyme that converts phenylalanine (Phe) to tyrosine. This causes the build-up of Phe in the body and, in particular, the brain, leading to significant neurocognitive symptoms. The primary current treatment for most of the PKU population is a restrictive and unpalatable low-Phe diet, and — especially in adolescence and adulthood — compliance is typically poor. No enzyme therapy for PKU is yet commercially available, although a daily injectable product is in clinical development. Codexis set out to develop an orally stable enzyme for the treatment of PKU in the gastrointestinal (GI) tract.
The challenge with developing an oral, enzyme-based treatment is the need for the enzyme to function in the upper GI tract where Phe is liberated from protein in food, and where systemic Phe is recirculated. It is important that Phe be effectively degraded before it finds it way (back) to the blood stream and the periphery of the body. Over 100s of millions of years the upper GI tract has evolved to become highly effective at digesting protein, and therefore it is a highly adverse environment for therapeutic enzymes and biologics in general. Using its CodeEvolver® technology, Codexis was able to engineer novel enzyme variants that can withstand proteolytic attack.
In vitro studies demonstrated that these enzyme variants retain significant levels of activity after exposure to trypsin and chymotrypsin for 10 hours in vitro, while the natural enzyme retains no activity. In vivo animal data are very promising, with demonstration of conversion of significant quantities of Phe under the study conditions. In addition, the enzyme variants are readily manufactured via E. coli fermentation, and Codexis has pending patent applications in many countries, covering a diverse set of such enzymes, their production, and use.
Expanding Pipeline
In addition to the lead candidate for PKU treatment, Codexis has a compound at the lead optimization stage for a different indication that involves extending the half-life of the enzyme in both serum and tissue and, at the same time, reducing its immunogenicity. In three other disease areas, Codexis has candidates at the hit identification stage where the challenges presented require proteins with novel functionality, tissue-specific targeting capabilities, and improved biodistribution.
Delivery of proof-of-concept for these enzyme therapeutics will not only create a healthy pipeline of commercial partnering opportunities, it will also further demonstrate the general utility of the CodeEvolver® platform technology to develop superior biotherapeutics.
There is significant value for protein engineering across other classes of biotherapeutics, including antibodies, antibody-drug conjugates and fusion proteins.
Conclusion
Advanced protein engineering technologies developed by Codexis have significant potential in discovery and development of more effective biologic drugs that address significant unmet medical needs. The CodeEvolver® platform can be applied in a number of ways to address significant development challenges, ranging from lead identification and optimization to resurrecting old biologic drug candidates.
Because of the ability to modify protein function and screen in a very targeted way against properties specific to the end-use application, enzymes and proteins developed using Codexis’ unique protein engineering technologies benefit not only from improved properties, but have the potential to exhibit novel functionalities that can overcome specific metabolic deficiencies not addressable with typical enzyme replacement therapies. As a result, Codexis’ approach to the development of new biologic drug substances represents an exciting advancement in the application of protein engineering to biotherapeutic drug development.
Since passage of the Orphan Drug Act in 1983, interest in the development of treatments for rare diseases has risen dramatically. A number of enzyme replacement therapies (ERTs) have been launched or are currently in development, with ongoing investment in these treatments anticipated. According to experts in the field, however, therapeutic enzymes currently on the market are not as effective as desired and typically do not completely resolve the symptoms they are intended to treat due to a variety of issues. Codexis is applying its expertise in protein engineering to design more effective ERTs and other biologic drug substances.

